Sunscreens, plastics and paints often contain zinc oxide nanoparticles, which protect skin and other surfaces from harmful ultraviolet rays. The advantage of nanoparticles is that we can’t see them on our skin, unlike larger particles that appear white.
In response to recent claims that nanoparticles of zinc oxide could increase the risk of sunlight damaging the skin if they were absorbed into the skin, Australian researchers are using synchrotron techniques to explore the detailed toxicology and reactivity of zinc oxide.
Their main aims are to help ensure that zinc oxide nanoparticles are safe for human use and don’t adversely affect the environment, and that legislation governing the use of nanoparticles is based on accurate scientific data.
Melbourne scientist Terry Turney is working with industry, CSIRO, Monash, RMIT and Deakin Universitiesy and medical researchers to determine the long-term impact of zinc oxide nanoparticles in sunscreens. As a former director of nanotechnology for CSIRO, Terry and his research group helped developed an ultra-low-cost method for producing nanoparticles, now used by a Melbourne company that exports tonnes of precisely engineered zinc oxide nanoparticles around the world. His current interests include a commercial company that makes micronutrients for agriculture.
Zinc oxide is a photocatalyst. When it absorbs light energy, it can then act as a catalyst, i.e. zinc oxide helps to promote other reactions such as oxidation without actually undergoing any reactions itself. Although the effect is more pronounced in smaller particles such as nanoparticles, it is not clear whether the increased reactivity is entirely due to the increased relative surface area found in nanoparticles.
Terry and his colleagues are looking closely at how introducing very small quantities of cobalt or manganese atoms (either inside the nanoparticles or on the surface) might influence zinc oxide nanoparticle properties. A single nanoparticle is typically 20-30 nm across and might contain around 200 cobalt or manganese atoms, so high-precision analysis is essential. The position and number of the cobalt or manganese atoms can be controlled by modifying the manufacturing conditions.
Synchrotron techniques make it possible to characterise the positions of the cobalt and manganese atoms even when these are present in very small numbers.
“We’re starting to think of the Australian Synchrotron as a one-stop shop,” Terry says. “We’ve already used three beamlines: soft x-ray absorption, x-ray absorption spectroscopy and powder diffraction. And yes, we can see the difference between samples from different manufacturing processes.
“Using the synchrotron, we’ve finally been able to obtain precise structural details of our relatively large nanoparticles (20-30 nm). Medical researchers are now correlating that data with chemical and biological reactivity studies.
“Next year we’d like to use the x-ray fluorescence microprobe to look at where the nanoparticles might end up inside the skin cells.”
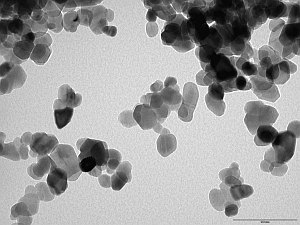
Electron micrograph of zinc oxide nanoparticles. The scale bar is 100 nm.
