28 June 2013
Researchers are making increasing use of correlative microscopy, where the same sample is subjected to two or more complementary techniques in a way that enables data from the separate techniques to be accurately combined for each precise location on the sample.
The trend was highlighted recently in a Nature technology article by Caitlin Smith (Nature, 13 December 2012, 492, 293-7), which featured a photo of the Maia detector on the XFM beamline and quoted Australian Synchrotron Interim Director Andrew Peele.
“Rather than just correlative imaging, the goal is to combine a whole suite of characterisation methods into something that might better be described as multimodal microscopy,” Andrew said.
The intense beams of light generated by the Synchrotron “can be used for imaging experiments that may include x-ray fluorescence, x-ray diffraction, light microscopy, electron microscopy, tomography or infrared microscopy”.
“One of the challenges we face is comparing information across widely different techniques,” Andrew said, “especially those with quite different resolutions, such as fluorescence data and high-resolution diffraction data.”
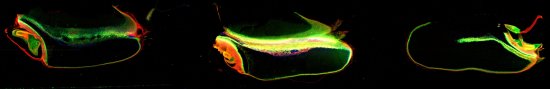
For example, x-ray fluorescence microscopy (XFM) is one synchrotron technique that is ideal for correlative microscopy.
The XFM images above show the concentration and distribution of manganese (red) and zinc (green) and copper (blue) in single wheat grains. They were acquired as part of a research project investigating bio-fortification of essential trace elements in cereal grains.
Data courtesy of Babasola Ajiboye, Sam Stacey, and Mike McLaughlin, Agriculture, Food and Wine, University of Adelaide. The seeds were scanned simultaneously along with three others to produce an image (50.6 x 4.9 mm; 25300 x 2448 pixels, 62 M pixels). Velocity of 8.192 mm/s, dwell 0.244 ms/pixel, pixel size 2 microns. Total scan time ~4.5 hours. Pixels/sec = 4100.
