- Details
PHD student Dr Leonie van ‘t Hag has been awarded the prestigious 2017 ANSTO, Australian Synchrotron Stephen Wilkins Medal for her PhD thesis.

The award recognises her research to improve the method to crystallise proteins and peptides in order to study their structure, using a technique called crystallography.
“Leonie’s insights into crystallisation processes could significantly help the development of treatments for a variety of illnesses,” said Australian Synchrotron Director, Professor Andrew Peele.
Most solid material in the world is made of crystalline structures. Crystals are made up of rows and rows of atoms or molecules stacked up like boxes in a warehouse, in different arrangements.
The science of determining these atomic or molecular structures from crystalline materials is called crystallography.
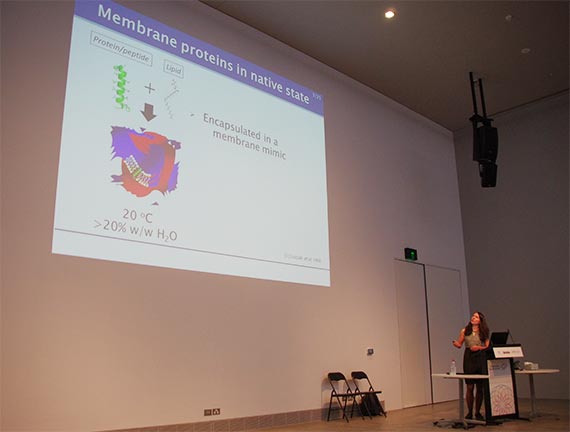
The Stephen Wilkins Medal is awarded annually to a PhD student at an Australian or New Zealand University who is judged to have completed the most outstanding thesis of the past two years.
Professor Peele said Dr van ’t Hag, who while working at the University of Melbourne, CSIRO and RMIT University was a prolific user of ANSTO facilities at the Australian Centre for Neurtron Scattering, the National Deuteration Facility and the Australian Synchrotron, was thoroughly deserving of her medal.
The type of crystallisation explored by Dr van ‘t Hag is one of a number of methods used to create 3D structures of proteins, including membrane proteins that are typically very difficult to crystallise.
“The application of this technique can lead more success in solving the 3D structures of proteins, which are important for rational drug design for a wide range of diseases,” Professor Peele said.
“I congratulate Dr van ’t Hag for her work in this field and on behalf of the Wilkins family and ANSTO. A contribution of $3000 will also be awarded to support her further career development.”
Dr Mike James, who is the Head of Science at The Australian Synchrotron, congratulated Dr Leonie, and said she was most deserving of the award and the recognition.
“Steve Wilkins was a friend and mentor to many young researchers, so it’s fitting the ANSTO, Australian Synchrotron annual thesis medal be named in his honour,” said Dr James.
“Leonie is certainly a very worthy recipient, and we are very excited to see where she will go with her career, and what benefits and impacts the application of her research will bring.”
The award is named in honour of Stephen (“Steve”) Wilkins, a widely-respected and internationally-renowned X-ray scientist, who made pioneering contributions in many areas of X-ray science and optics.
To be eligible, work is required to be undertaken at and acknowledge, the Australian Synchrotron, or work undertaken at international synchrotron facilities and funded by various ANSTO access programs.
Dr Leonie van ‘t Hag will be attending the event presentation ceremony to present her thesis and receive the award from Mrs Linda Wilkins – wife of the late Stephen Wilkins.
Media contact: Phil McCall 0438 619 987
- Details
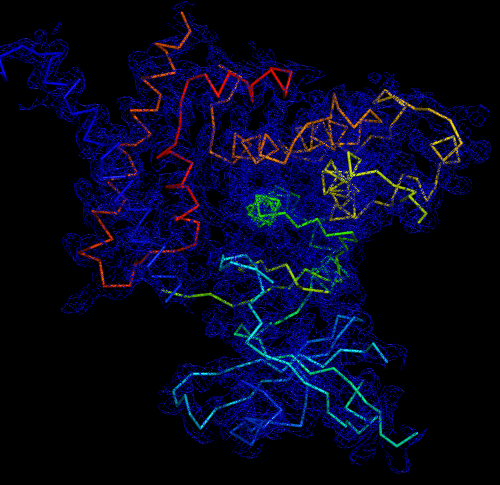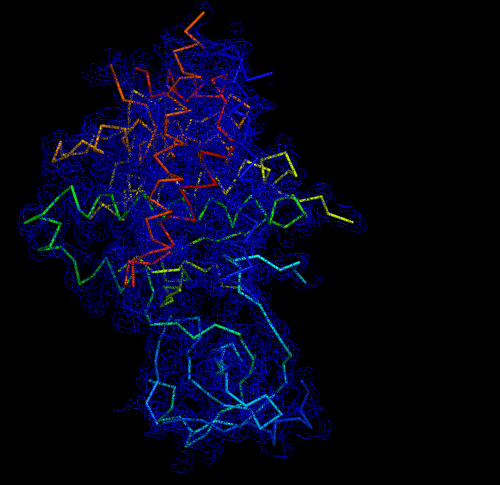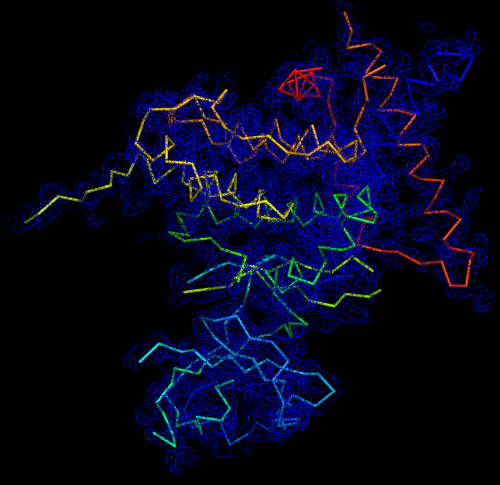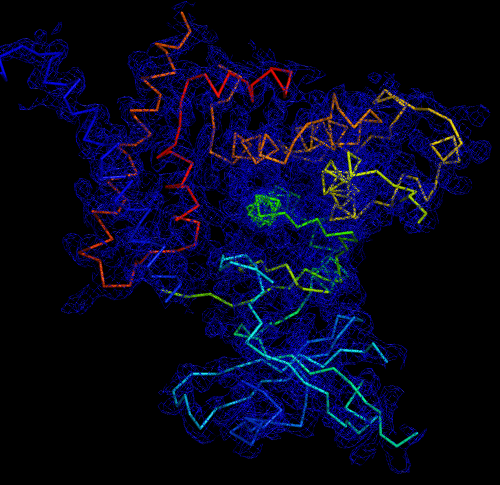
Melbourne researchers have used the Australian Synchrotron to produce the first three-dimensional structure of a molecular scaffold, known to play a critical role in the development and spread of aggressive breast, colon and pancreatic cancer.
Armed with the structure, the research team is looking at ways of targeting parts of the scaffold molecule critical for its function. They hope the research will lead to novel strategies to target cancer.
The research was the result of a long-standing collaboration between Walter and Eliza Hall Institute (WEHI) researchers Dr Onisha Patel and Dr Isabelle Lucet and Monash University Biomedical Research Institute researcher Professor Roger Daly.
Dr Santosh Panjikar, a macromolecular crystallographer at the Australian Synchrotron and Dr Michael Griffin from Bio21 Institute at the University of Melbourne made important contributions to the study, which was published in the journal Nature Communications.
Lucet said SgK223 is a member of a family of proteins called pseudokinases and had been classified for a long time as a ‘dead enzyme’.
“SgK223 doesn’t have the measurable activity that we see with other types of enzymes, and this meant it was largely ignored. However in the past decade, we’ve come to understand that this ‘dead enzyme’ plays an active and important role in cell signalling,” Lucet said.
MX2 beamline used to determine crystal structure
Panjikar explained that measurements on the macromolecular crystallography beamline, MX2 and small angle X-ray scattering on the SAXS/WAXS beamline were used to determine the crystal structure of SgK223 and oligomeric state of SgK223 in solution respectively.
“There were many challenges working on this protein,” said Panjikar.
Initial experiments on crystals of SgK223 were not successful because the protein is highly sensitive to various heavy atom solutions, and to X-ray radiation and deteriorates very quickly.
“Protein crystals are normally sensitive to radiation but the selenomethionine protein crystals were more sensitive,” said Panjikar.
“You want to collect your data in a way that doesn’t damage the crystal but retains the anomalous signal,” said Panjikar.
"We designed a diffraction strategy for the sample, in which we used a small sized beam 20 microns across and clad at several places going from one position to the next on the rod-shaped crystals.”
The researchers collected multiple data sets at different X-ray energies around selenium edge for multiple wavelength anomalous dispersion (MAD), a specialised technique that allows them to use ‘tunable energy’.
“Selenium has an absorption edge at particular energy where it absorbs more X-rays. We went on to collect X-ray data from Sgk223 crystal at the higher energy side of that edge and also at below the edge,” explained Panjikar.
They were able to solve the crystal structure using a software pipeline, Auto Rickshaw, developed by Panjikar.
“Where you have multiple data sets at different energies, you need to check which data set will actually work. In this case, with Auto Rickshaw we found one combination of the data sets that worked very well,” said Panjikar.
The data was used to get the preliminary phases and electron density map, which enabled the researchers to build the 3D model.
“When you determine the crystal structure of the protein you know what the molecule looks like but it also confirms if the molecule is a monomer or dimer,” said Panjikar.
SAX confirms crystal structure
“We could see that SgK223 was a dimer, but needed supporting confirmation that what we saw in the crystal structure was the same in solution. “
Validation of the dimer was achieved using small angle X-ray scattering of the native protein.
Other biochemical techniques carried out at WEHI, Monash and the University of Melbourne were used in the study.
“The world-class facilities at the Australian Synchrotron in Melbourne were instrumental in the discovery,” co-author Lucet said.
“It is the only facility in the Southern Hemisphere that has the specialised technology required to provide us with detailed knowledge essential for seeing molecules at an atomic level. This is essential if we wish to discover and develop drugs that target and interfere with molecules that drive cancer and other diseases.”
Media release
Read more about the research on the Walter and Eliza Hall Institute of Medical Research.
DOI: 10.1038/s41467-017-01279-9
- Details

Prof Andrew Peele, Director of Australian Synchrotron (above left) and Dr Richard Garrett, Senior Advisor Synchrotron Science, Strategic Projects, Industry and External Engagement (above centre) attended the 20th anniversary of user operations at the SPRing-8 synchrotron, in Hyōgo Prefecture, Japan last week.
They were among 500 guests, industry leaders and politicians from Japan, and directors of the majority of the world's major synchrotrons, who gathered at the historic Himeji Castle also in Hyōgo Prefecture to mark the occasion.
There were photographs on display of all the synchrotrons that sent directors or representatives to the event.
“It was an opportunity for those of us who manage synchrotron facilities to congratulate SPRing-8 on the milestone and get together to discuss the application of synchrotron science to meet future challenges,” said Prof Peele.
“Synchrotron science using X-rays and infrared light continues to be a powerful and invaluable tool in investigating the nature of materials for practical applications.”
The SPRing-8 synchrotron, the world’s largest 3rd generation synchrotron opened to national and international users from industry, academia and government in 1997. The synchrotron radiation facility operates with a beam energy of 8 GeV with 62 beamlines.
Third-generation synchrotron radiation facilities are designed especially for installing as many insertion devices as possible in a dedicated storage ring.
The anniversary ceremony was followed by a symposium on "Synchrotron Radiation for the Future of Humanity", which held in the 17th century castle.
The Australian Consul General at the Osaka Consulate, David Lawson (above right) , also attended.
ANSTO has an international partnership with the SPRing-8 synchrotron.
